Sponsored by AbbVie Medical Affairs and Ironwood Pharmaceuticals Intended for Physicians
Chapter 1
Diagnosis and Management of Pediatric Functional Constipation and Adult Chronic Idiopathic Constipation
Chapter 2
The Impact of Seamless Care Transition on Patient Outcomes
Chapter 3
Navigating Barriers and Individualizing the Transition of Care
Chapter 4
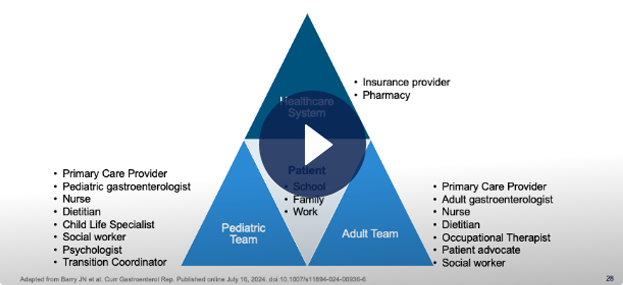
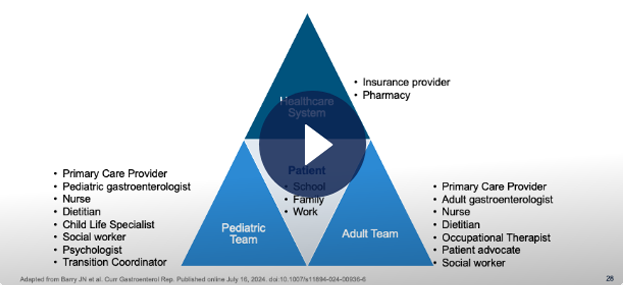
Understanding an Ideal Transition Process and Enhancing Continuity of Care Through a Multi-Disciplinary Approach
Linaclotide Indications, Important Safety Information, and Boxed Warning
Linaclotide is indicated for the treatment of:
- Irritable bowel syndrome with constipation (IBS-C) in adults
- Chronic idiopathic constipation (CIC) in adults
- Functional constipation (FC) in pediatric patients 6 to 17 years of age
IMPORTANT SAFETY INFORMATION AND BOXED WARNING
WARNING: RISK OF SERIOUS DEHYDRATION IN PEDIATRIC PATIENTS LESS THAN 2 YEARS OF AGE.
Linaclotide is contraindicated in patients less than 2 years of age; in nonclinical studies in neonatal mice, administration of a single, clinically relevant adult oral dose of linaclotide caused deaths due to dehydration.
Contraindications
- Linaclotide is contraindicated in patients less than 2 years of age due to the risk of serious dehydration.
- Linaclotide is contraindicated in patients with known or suspected mechanical gastrointestinal obstruction.
Warnings and Precautions Risk of Serious Dehydration in Pediatric Patients Less Than 2 Years of Age
- Linaclotide is contraindicated in patients less than 2 years of age. In neonatal mice, linaclotide increased fluid secretion as a consequence of age-dependent elevated guanylate cyclase (GC-C) agonism, which was associated with increased mortality within the first 24 hours due to dehydration. There was no age-dependent trend in GC-C intestinal expression in a clinical study of children 2 to less than 18 years of age; however, there are insufficient data available on GC-C intestinal expression in children less than 2 years of age to assess the risk of developing diarrhea and its potentially serious consequences in these
patients.
Diarrhea
- In adults, diarrhea was the most common adverse reaction in Linaclotide-treated patients in the pooled IBS-C and CIC double-blind placebo-controlled trials. The incidence of diarrhea was similar in the IBS-C and CIC populations. Severe diarrhea was reported in 2% of 145 mcg and 290 mcg Linaclotide-treated patients and in <1% of 72 mcg Linaclotide-treated CIC patients. In pediatric patients 6 to 17 years of age, diarrhea was the most common adverse reaction in 72 mcg Linaclotide-treated patients in the FC double-blind placebo-controlled trial. Severe diarrhea was reported in one Linaclotide-treated patient. If severe diarrhea occurs, dosing should be suspended and the patient rehydrated.
Common Adverse Reactions (incidence ≥2% and greater than placebo)
- In IBS-C or CIC adult patients: diarrhea, abdominal pain, flatulence, and abdominal distension.
- In FC pediatric patients: diarrhea.
Review accompanying linaclotide full Prescribing Information for additional information, visit www.rxabbvie.com or contact AbbVie Medical Information at 1-800-633-9110.
Chapters
Coming up next in 0:00
Watched
Placeholder
Diagnosis and Management of Pediatric Functional Constipation and Adult Chronic Idiopathic Constipation
Chapter 1 | 21m 15s


Coming up next in 01:05
Watched
Placeholder
The Impact of Seamless Care Transition on Patient Outcomes
Chapter 2 | 20m 29s


Coming up next in 00:00
Watched
Placeholder
Navigating Barriers and Individualizing the Transition of Care
Chapter 3 | 20m 21s
Coming up next in 00:00
Watched
Placeholder
Understanding an Ideal Transition Process and Enhancing Continuity of Care Through a Multi-Disciplinary Approach
Chapter 4 | 19m 28s


Program Objectives
Review the diagnosis and management of functional constipation (FC) and overlapping symptoms in chronic idiopathic constipation (CIC).
Discern the barriers that interfere with the transition from pediatric to adult care.
Understand the importance of a seamless transition of care and its impact on patient outcomes.
Discuss a multi-disciplinary approach to improve continuity of care.
Clinical Perspectives Presented by:

Joy Liu, MD
Feinberg School of Medicine
Northwestern University

Neha Santucci, MD, MBBS
Disorders of Gut-Brain
Interaction Program
Cincinnati Children’s Hospital

Kristina Skarbinski, NP
Gastroesophageal Surgery
Program – Massachusetts
General Hospital

 Download Transcript
Download Transcript